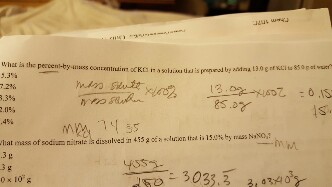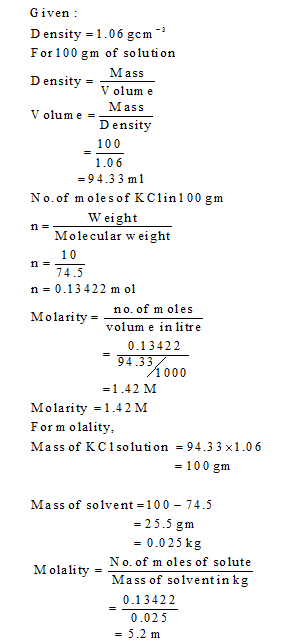Calculate the molarity and molality of 20% aqueous ethanol (C2H5OH) solution by volume. (Density of solution = 0.96 g/mL)

An aqueous solution contains 30% w/v of urea , density of solution is 1.2 g/ml. Calculate mass of water in 100ml solution.

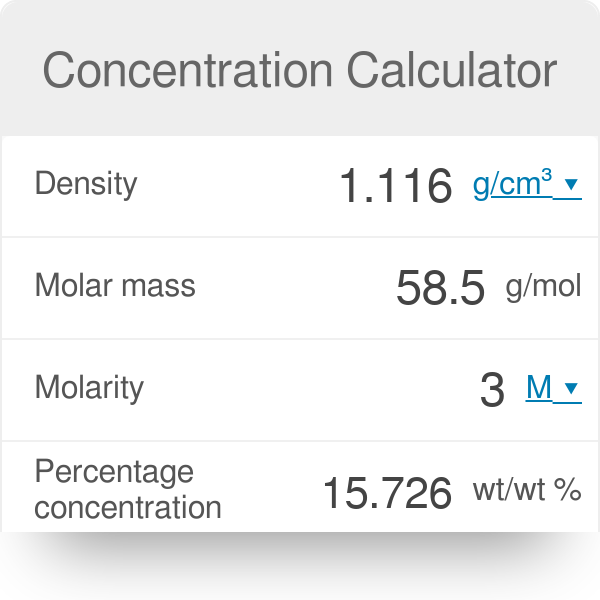
The density of 3 M solution of NACl is `1.25 g mL^(-1)`. Calculate the molality of the solution. - YouTube

Ketamine decreases neuronally released glutamate via retrograde stimulation of presynaptic adenosine A1 receptors | Molecular Psychiatry
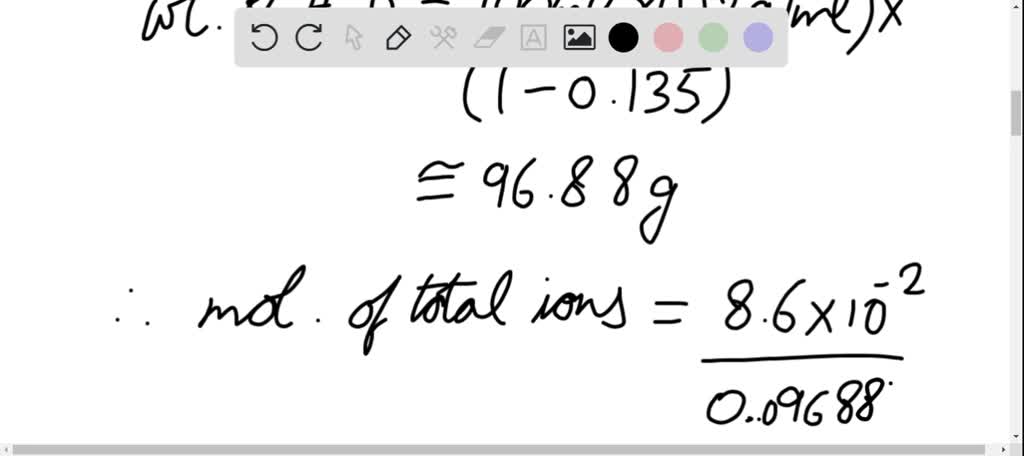
SOLVED:A 100.0 -mL aqueous sodium chloride solution is 13.5% NaCl by mass and has a density of 1.12 g / mL . What would you add (solute or solvent) and what mass

Solution Density Models as Functions of Sodium Chloride, Hydroxypropyl-β-cyclodextrin, and Temperature (278.15–333.15 K) via Progressive Linear and Stepwise Regression | Journal of Chemical & Engineering Data

Cells in New Light: Ion Concentration, Voltage, and Pressure Gradients across a Hydrogel Membrane | ACS Omega

The density of `3M` solution of `NaCl` is `1.25 g mL^(-1)`. The molality of the solution is... - YouTube

The density of a 10.0% by mass of KCl solution in water 1.06 g/mL. Calculate molarity, molality and mole fraction of KCl in this solution respectively.

Quantification of Zeta-Potential and Electrokinetic Surface Charge Density for Colloidal Silica Nanoparticles Dependent on Type and Concentration of the Counterion: Probing the Outer Helmholtz Plane | The Journal of Physical Chemistry C
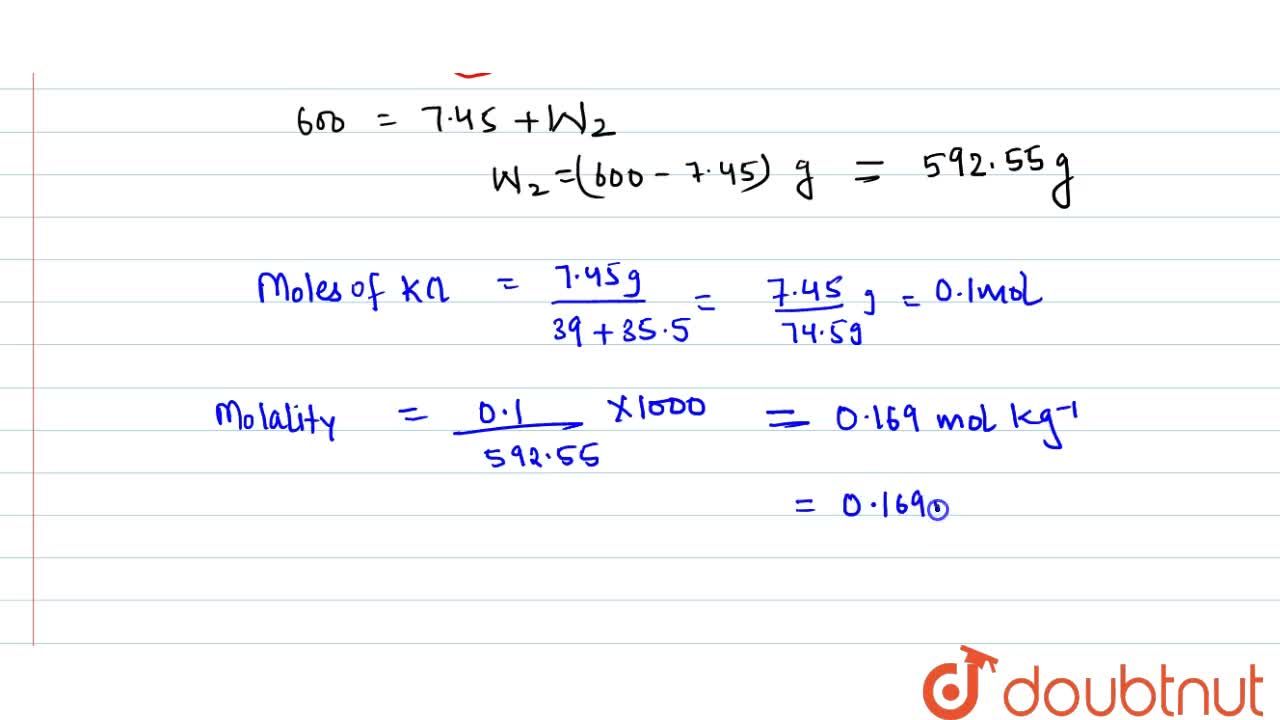
Calculate the molarity of KCl solution prepared by dissolving 7.45 g of KCl in 500 mL of the solution. (d(sol) = 1.2 g mL^(-1))
SOLVED: What is the Molar concentration of a solution with a volume 3.3 mL that contains 12 grams of ammonium sulfite? How many grams of copper (II) fluoride are needed to make

Utilization of Low-Concentration CO2 with Molecular Catalysts Assisted by CO2-Capturing Ability of Catalysts, Additives, or Reaction Media | Journal of the American Chemical Society

Aq KCL #solution of #density 1.2 g/ml has a #molality of 3.30 mol/kg. find #molarity. #jeemains2021 - YouTube